The flow battery is revolutionizing the concept of grid energy storage by uniquely combining fluid dynamics with electrochemical technology. This innovative system allows for efficient energy management, making it increasingly popular in renewable energy applications and home energy solutions. For those interested in exploring this advanced technology, there are comprehensive battery design instructions available specifically for building a zinc-iodide flow battery. This type of flow battery uses an electrochemical cell divided into two sections, enhancing the overall performance and longevity. With the rise of DIY flow battery projects, enthusiasts can now engage with sustainable energy solutions while contributing to the growing community focused on energy independence.
Also known as redox flow batteries, these systems store energy in liquid solutions, making them particularly effective for large-scale applications such as renewable energy storage. The unique construction of the zinc-iodide flow battery allows for easy scalability and manageable operational costs, which is crucial for advancing green technologies. By utilizing various methods, including DIY projects, individuals can create their own energy storage devices based on open-source designs and readily accessible materials. This approach not only fosters innovation but also empowers users to contribute to the development of sustainable energy systems. As the demand for reliable storage solutions increases, the role of flow batteries in modern energy infrastructure becomes more significant.
Understanding Flow Batteries for Energy Storage
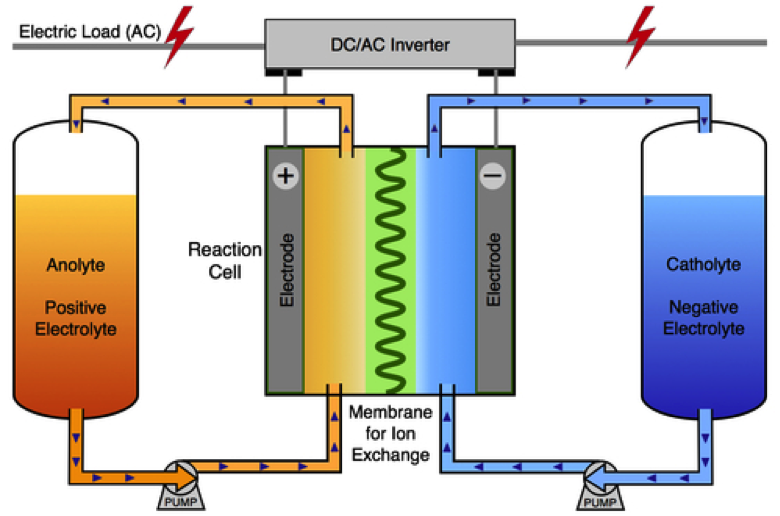
Flow batteries represent a cutting-edge solution in the realm of energy storage, marrying the principles of electrochemistry with fluid dynamics. Designed primarily for grid energy storage applications, these batteries utilize two electrolyte solutions that flow through an electrochemical cell, enabling them to store and release energy efficiently. Their ability to maintain long cycle life and high energy capacity makes them particularly suitable for renewable energy integration, helping to bridge the gap between energy generation and consumption.
The unique structure of flow batteries allows for scalable energy capacities determined by the size of the storage tanks. As advancements in battery technology continue, flow batteries stand out due to their ease of maintenance and performance predictability. Innovations like the zinc-iodide flow battery exemplify how materials and design can enhance efficiency, making these batteries a vital part of sustainable energy infrastructures.
Building Your Own DIY Flow Battery
For those intrigued by the details of actualizing a flow battery, the DIY flow battery project has garnered interest among hobbyists and energy enthusiasts. Following the design instructions published by the Flow Battery Research Collective, anyone can take on the challenge of constructing a zinc-iodide flow battery. The guide highlights the importance of choosing the right materials and components, such as brass-backed grafoil for current collection and 3D-printed polypropylene for robust reservoir construction.
Embarking on a DIY project not only fosters a deeper understanding of battery technologies but also offers the opportunity to engage with a community of like-minded DIYers. In addition to adhering to safety protocols—such as testing for leaks with distilled water before full assembly—individuals can share their builds, troubleshoot issues, and explore improvements in battery design through various online forums and communities dedicated to flow battery research and development.
Components of the Zinc-Iodide Flow Battery
The zinc-iodide flow battery is crafted using specific materials to optimize its performance and longevity. Central to its structure is the electrochemical cell, which houses the reaction that occurs during both charging and discharging processes. The apparatus includes two separate compartments, each containing an electrolyte solution. The use of graphite felt as a porous electrode and matte photo paper for the membrane ensures effective ion exchange while maintaining structural integrity.
In addition to the electrodes and membranes, the configuration of current collectors made from compressed graphite sheets allows for efficient electron flow, enhancing the overall energy conversion process. The choice of zinc chloride and potassium iodide as the primary components of the electrolyte solution is crucial as they facilitate the reversible chemical reactions that define battery operation. This combination not only provides a stable platform for energy release but also promotes a rapid recharge capability, essential for cyclical energy demands.
Operating Principles of Flow Batteries
A flow battery operates on the principle of electrochemical reactions occurring between the electrolyte solutions in the cell. Each charging cycle converts electrical energy into chemical energy, represented by the deposition of zinc on the cathode, while iodine ions are generated in the anode compartment. Understanding these processes is key to optimizing the efficiency and effectiveness of the flow battery.
During discharge, the reverse occurs; the zinc re-dissolves, supplying electrons to the external circuit, while iodine ions are reduced back to iodides. This ability to efficiently convert and store energy makes flow batteries an attractive choice for sustaining energy releases over time, essential for balancing supply and demand in grid energy storage scenarios.
Safety Precautions in Building Flow Batteries
When constructing a DIY flow battery, safety must be a top priority due to the chemical nature of the components involved, especially when working with zinc chloride and potassium iodide solutions. Proper protective equipment, such as gloves and goggles, should be used to mitigate any risks associated with spills or corrosive exposure. Before introducing the electrolyte into the cell, it’s advisable to conduct thorough checks for any leaks, particularly with distilled water, to prevent potential staining and chemical hazards.
Another aspect of safety involves the maintenance of the electrical components, especially the Arduino controller and pump systems. Proper insulation and securing of electrical connections are vital in preventing short circuits or malfunctions that could pose safety risks during operation. By taking these precautions, builders can create a functional and safe DIY flow battery system.
Advantages of Using Flow Batteries for Grid Storage
Flow batteries offer several advantages that make them uniquely suited for grid energy storage solutions. Unlike traditional batteries, flow batteries can provide extended discharging capabilities without significant degradation in performance. Their modular design enables scaling of energy capacity, which is particularly beneficial for energy demanding applications such as renewable energy fluctuations.
Moreover, flow batteries are characterized by their longer life cycles and lower operational costs, thanks to their ability to be cycled multiple times without substantial wear. This long-term reliability can lead to reduced replacement frequency and improved overall efficiency for grid applications, solidifying their role as a critical component in the advancement of sustainable energy technologies.
Future Prospects for Flow Battery Technology
As the demand for innovative energy solutions grows, the future of flow battery technology appears promising. Research into new materials and improved electrolytes continues to evolve, paving the way for enhanced performance in energy density and efficiency. Innovations in battery design, such as more accessible DIY kits and open-source blueprints, are likely to engage a broader audience in the field of renewable energy.
Additionally, as industries seek to decarbonize their operations, the integration of flow batteries into existing grid infrastructures can play a pivotal role in stabilizing energy supply from renewable sources like wind and solar. With ongoing advancements and increased adoption, flow batteries are positioned to become a foundational technology in the quest for sustainable and resilient energy systems.
Comparing Flow Batteries to Traditional Batteries
When comparing flow batteries to traditional lithium-ion batteries, one of the most significant differences lies in their operational mechanics. While lithium-ion batteries store energy chemically within their cells, flow batteries utilize external tanks of liquid electrolyte, allowing for unique scalability and customizable energy output. This differentiation results in longer cycle life and less degradation over time in flow batteries.
Moreover, flow batteries can be discharged to zero without severe consequences to their lifespan, unlike traditional batteries, which often suffer from shorter longevity when consistently pushed to depletion. This aspect makes flow batteries particularly advantageous for long-term energy storage solutions needed in modern energy infrastructures.
The Role of Electrochemical Cells in Flow Battery Design
At the heart of every flow battery lies the electrochemical cell, which serves as the site for energy conversion. In the case of the zinc-iodide flow battery, the design intricately allows for two solutions to flow past each other, facilitating ion transfer and electron flow through the electrodes. The efficiency of this process plays a crucial role in determining the battery’s overall performance.
Proper design of the electrochemical cell also influences its scalability and operational stability. By utilizing durable materials and leveraging innovative design techniques, builders can enhance the effectiveness of energy storage while ensuring longevity across multiple charge cycles. As researchers refine these designs, we can expect to see improvements that further push the boundaries of flow battery technology.
Frequently Asked Questions
What is a flow battery and how does it work?
A flow battery is an electrochemical energy storage system that combines the flow of electrolytes with electronic flow to store and release energy. It typically consists of two separate electrolyte reservoirs that circulate through an electrochemical cell, where chemical reactions take place to store energy during charging and release it during discharging.
How can I build my own DIY flow battery?
To build a DIY flow battery, you can follow the design and assembly instructions provided by the Flow Battery Research Collective. Their guide includes steps to construct a small zinc-iodide flow battery, highlighting the use of components like 3D-printed parts, brass-backed grafoil current collectors, and a simple Arduino-based control system.
What materials do I need for a zinc-iodide flow battery?
Building a zinc-iodide flow battery requires materials such as zinc chloride, potassium iodide, brass-backed grafoil for current collectors, graphite felt for electrodes, and polypropylene for the cell frame and reservoir tanks, all of which are designed to enhance chemical resistance.
What are the advantages of using flow batteries for grid energy storage?
Flow batteries offer several advantages for grid energy storage, including scalability, long cycle life, and the ability to decouple energy storage from power output. This makes them suitable for stabilizing electricity supply by storing excess energy generated from renewable sources.
Can I regulate the charge and discharge cycles of my flow battery?
Yes, you can regulate the charge and discharge cycles of your flow battery by using an open-source potentiostat, along with an Arduino system that controls the peristaltic pumps and monitors the electrolyte flow, ensuring optimal operation of the battery.
What precautions should I take when testing a DIY flow battery for leaks?
Before introducing the electrolyte into your DIY flow battery, it is crucial to test the system for leaks using distilled water. This is especially important with zinc-iodide systems, as iodide ions can cause staining and indicate potential leakage.
What is the role of electrolyte in a zinc-iodide flow battery?
In a zinc-iodide flow battery, the electrolyte, which consists of zinc chloride and potassium iodide, plays a key role in facilitating the electrochemical reactions that charge and discharge the battery. During charging, zinc ions deposit onto the cathode, while iodine and similar ions accumulate at the anode.
Are there alternative designs for DIY flow batteries?
Yes, in addition to the zinc-iodide flow battery, there are several alternative designs for DIY flow batteries that can be created, often focusing on different electrolytes or configurations to achieve high energy density and improved performance.
| Key Point | Details |
|---|---|
| Introduction to Flow Battery | Flow batteries combine electronic flow and fluid flow for energy storage. |
| Design and Assembly | Published instructions for a small zinc-iodide flow battery by the Flow Battery Research Collective. |
| Components of the Battery | Central electrochemical cell with two separate sections, each having a reservoir and a peristaltic pump. |
| Materials Used | Brass-backed grafoil as current collectors, graphite felt electrodes, matte photo paper as separator, and 3D-printed polypropylene frame. |
| Operation of the Cell | Uses an open-source potentiostat managed by an Arduino for charge/discharge cycles. |
| Electrolyte Composition | Zinc chloride and potassium iodide are the main components of the electrolyte. |
| Charging and Discharging Process | Zinc deposits on the cathode during charging; reverses during discharging. |
| Safety Recommendations | Test for leaks with distilled water due to iodide ion staining potential before using electrolyte. |
| Community and Support | A forum is available for documenting progress and seeking help in building flow batteries. |
| Variability in Designs | Many DIY flow cell options with varying energy densities available. |
Summary
Flow battery technology represents a remarkable advancement in grid energy storage solutions. By integrating both electronic and fluid dynamics, flow batteries offer unique advantages over traditional battery systems. The design and assembly of a zinc-iodide flow battery, as presented by the Flow Battery Research Collective, showcase innovative materials and engineering principles that enhance chemical resistance and efficiency. With the proper guidance and community support, anyone interested can explore the potential of flow batteries, contributing to the evolution of renewable energy storage.